| ALLYL ISOTHIOCYANATE | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 57-06-7 107231-30-1, 50888-64-7, 50978-48-8, 58391-87-0 (former numbers), 8007-40-7 (Mustard oil) |
|
| EINECS NO. | 200-309-2 | |
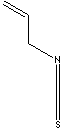
| FORMULA | CH2=CHCH2NCS | |
| MOL WT. | 99.15 | |
|
H.S. CODE |
||
| TOXICITY | ||
| SYNONYMS | Isothiocyanic acid, allyl ester; Mustard oil, synthetic; | |
| 3-Isothiocyanato-1-propene; 2-Propenyl isothiocyanate; Allyl isosulfocyanate; Allyl isosulphocyanate; Allyl mustard oil, synthetic; Allyl sevenolum; Allylisothiokyanat; Allylsenevol, Allylsenfoel; Allylsevenolum; Allyspol; Artificial mustard oil; Artificial oil of mustard; Isothiocyanate d'allyle; Isothiocyansaeureallylester; Oleum sinapis; | ||
| DERIVATION | ||
|
CLASSIFICATION |
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | Clear to pale yellow, oily liquid | |
| MELTING POINT | -80 C | |
| BOILING POINT | 148 - 153 C | |
| SPECIFIC GRAVITY | 1.015 - 1.025 | |
| SOLUBILITY IN WATER | Insoluble | |
| pH | ||
| VAPOR DENSITY | ||
|
AUTOIGNITION |
||
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
1.526 - 1.528 | |
| FLASH POINT | 46 C | |
| STABILITY | Stable under ordinary conditions. Moisture sensitive | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
|
Thiocyanate is a salt or ester of thiocyanic acid (HSCN). Aqueous solutions of thiocyanic acid , also called sulfocyanic acid, are very strong acids of the equilibrium mixture of thiocyanic and isothiocyanic. Thiocyanates are bonded through the sulfur(s) with the structure R-S-C��N or the isomeric R-N=C=S (isothiocyanates). Thiocyanates are bonded through the sulfur(s) which replace for the oxygen (O) atom. Thiocyanates are the sulfur analog of isocyanates. Organic as well as metallic thiocyanates [CuSCN, Ca(SCN)2, NaSCN, KSCN] are very versatile compounds. They have wide range of applications including manufacturing industrial chemicals, pharmaceuticals and pesticides. It is used in freezing solutions, fabric dyeing, electroplating, steel pickling, printing, and corrosion inhibitor against acid gases. It is used in photography industry as a stabilizer or accelerator. Allyl- is the prefix for the univalent organic group, -CH2=CHCH2. Allyl alcohol is an example, CH2=CHCH2OH, clear, pungent liquid, boiling at 96 C; soluble in water. It is prepared from allyl chloride by hydrolysis. Allyl compound, an alkene hydrocarbon, has a vinyl group, CH2=CH-, attached to a methylene -CH2. Because of the highly reactive double bond, allyl can undergo free radical addition to double bond which readily combine with themselves or other monomers to form homopolymers or co-polymers which are used in the production of coatings, adhesives and elastomers. In addition to free radical addition, allyl compounds can participate in a wide variety of reactions including electrophilic additions, allylic substitution and oxidation. Allyl, an unsaturated bond, imparts a characteristic odor in some compounds. An example is allyl isothiocyanate which is the main ingredient of black mustards. (white mustard consists principally of p-hydroxybenzyl isothiocyanate). Allyl isothiocyanate is called mustard oil. Powdered mustard has essentially no aroma until it combines to moisture. Naturally occurring allyl isothiocyanate is known to have anti-tumor and anti-oxidant properties by inducing the activity of phase II detoxification enzymes in the urinary bladder. Synthetic allyl isothiocyanate is used as a fumigant, counterirritant in ointments and plasters and as an intermediate to create a desired chemical compound through a series of chemical reactions, including in the field of fungicide, bactericide, wood preservative, pharmaceutical and plastic and rubber. |
||
| SALES SPECIFICATION | ||
|
APPEARANCE |
Clear to pale yellow, oily liquid | |
| ASSAY | 98.0% min | |
| SPECIFIC GRAVITY | 1.015 - 1.025 | |
| REFRACTIVE INDEX |
1.526 - 1.528 | |
| TRANSPORTATION | ||
| PACKING | 200kgs in drum | |
| HAZARD CLASS | 6.1 (Packing Group: II) | |
| UN NO. | 1545 | |
| OTHER INFORMATION | ||
| Hazard Symbols: T, Risk Phrases: 10-24/25-34-42/43,
Safety Phrases:
23-26-36/37/39-45 FEMA No. 2034 |
||